Page 11 - emap_newsletter_V2
P. 11
eMAP – electronic EHA Medical HemAtology Program
WATCH WATCH
DR. REECE’S IN-DEPTH PRESENTATION OF THIS DR. REECE’S THOUGHTS ON THE RELEVANCE OF
ABSTRACT (~6 MINUTES) THESE DATA TO CANADIAN PRACTICE (~2 MINUTES)
CLICK HERE FOR THE LINK TO THE FULL ABSTRACT
Ciltacabtagene Autoleucel, a BCMA-directed CAR T cell Therapy, in Patients with
Relapsed/Refractory Multiple Myeloma: 2-year Post-LPI Results from the Phase
1b/2 CARTITUDE-1 Study
Lin Y, et al. Presented at EHA 2022 Congress. Abstract P961.
The BCMA-directed CAR T cell therapy cilta-cel has
demonstrated early, deep, and durable responses in
heavily pretreated patients with RRMM in the phase 1b/2
CARTITUDE-1 study. The objective of this analysis is to
report landmark two years post-last-patient-in results from
the CARTITUDE-1 study with a longer duration of follow up of
27.7 months.
A total of 97 RRMM patients who had received =3 prior lines
of therapy or were double refractory were treated with cilta-
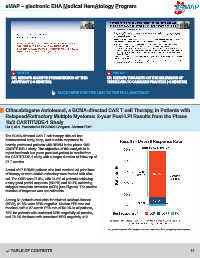
cel. The ORR was 97.9%, with 94.8% of patients achieving
= very good partial response (VGPR) and 82.5% achieving
stringent complete remission (sCR) (see Figure). The median
duration of response was not estimable.
Among 61 patients evaluable for minimal residual disease
(MRD), 91.8% were MRD-negative. Median PFS was not
reached, with a 27-month PFS rate of 54.9% in all patients,
73% for patients with sustained MRD negativity =6 months,
and 78.8% for those with sustained MRD negativity =12
PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
TABLE OF CONTENTS 11