Page 13 - emap_newsletter_V2
P. 13
eMAP – electronic EHA Medical HemAtology Program
Efficacy and Safety of ARI0002h, an Academic BCMA-directed CAR T cell Therapy
with Fractionated Initial Therapy and Booster Dose in Patients with Relapsed/
Refractory Multiple Myeloma
Fernandez De Larrea C, et al. Presented at EHA 2022 Congress. Abstract S103.
There are several CAR T cell products for RRMM in
development, including ide-cel that has recently been
Health Canada-approved, and cilta-cel, which is currently
under Health Canada review. ARI0002h is an academic
CAR T cell product with a humanized single chain
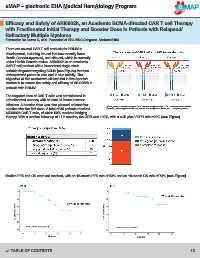
variable fragment targeting BCMA (see Figure) that has
demonstrated potent in vitro and in vivo activity. The
objective of this multicentre clinical trial in five Spanish
centres is to assess the safety and efficacy of ARI0002h in
patients with RRMM.
The targeted dose of CAR T cells was administered in
a fractionated manner, with at least 24 hours between
infusions. A booster dose was also planned at least four BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CD, cluster of differentiation;
months after the first dose. A total of 30 patients received Cilta-cel, ciltacabtagene autoleucel; Ide-cel, idecabtagene vicleucel; MM, multiple myeloma.
ARI0002h CAR T cells, of which 50% received bridging
therapy. With a median follow up of 17.5 months, the ORR was 100%, with a sCR plus VGPR rate >90% (see Figure).
Median PFS and OS were not reached, with an 18-month PFS rate of 53% and an 18-month OS rate of 73% (see Figure).
TABLE OF CONTENTS 13