Page 2 - emap_newsletter_V2
P. 2
eMAP – electronic EHA Medical HemAtology Program
SELECT ABSTRACTS ON CELLULAR THERAPY IN B-CELL LYMPHOMA
Lisocabtagene Maraleucel (Liso-cel) as Second-line Therapy for R/R Large B-cell
Lymphoma (LBCL) in Patients Not Intended for HSCT: Primary Analysis from the
Phase 2 PILOT Study
Sehgal A, et al. Presented at EHA 2022 Congress. Abstract S258.
Lisocabtagene maraleucel (liso-cel) is an autologous, cluster of differentiation (CD)19-directed CAR T cell product that demonstrated
superiority over standard of care (SOC), i.e., salvage chemotherapy followed by high-dose chemotherapy (HDCT) and hematopoietic
stem cell transplantation (HSCT), as second-line therapy in relapsed or refractory large B-cell lymphoma (R/R LBCL) patients
intended for transplant in the phase 3 TRANSFORM study. Those patients unable to undergo HDCT/HSCT due to advanced age,
comorbidity, or performance status have poor outcomes and limited treatment options. The objective of the prospective, open-label,
phase 2 PILOT study was to examine the efficacy and safety of liso-cel as second-line therapy for R/R LBCL in a group of patients
not intended for HSCT (see Transplant Not Intended Criteria).
Transplant Not Intended Criteria Details
Age =70 years
ECOG PS of 2
ECOG PS DLCO =60% adjusted for gender-specific hemoglobin concentration
LVEF <50%
Impaired pulmonary function CrCl <60 mL/min (calculated using Cockcroft-Gault)
AST/ALT >2 × ULN
Impaired cardiac function
Impaired renal function
Impaired hepatic function
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CrCl, creatinine clearance; DLCO, diffusing capacity of the lungs for carbon monoxide; ECOG PS,
Eastern Cooperative Oncology Group performance status; LVEF, left ventricular ejection fraction.
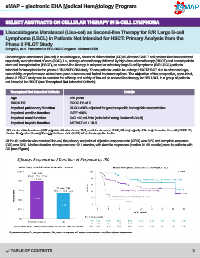
Of the 61 patients who received liso-cel, the primary endpoint of objective response rate (ORR) was 80% and complete response
(CR) was 54%. Median duration of response was 12.1 months, with durable responses (median 21.65 months) seen in patients with
CR (see Figure).
CI, confidence interval; CR, complete response; DOR, duration of response; IRC, independent review committee; ORR, objective response rate. 2
TABLE OF CONTENTS